Tumor-infiltrating lymphocyte (TIL) therapy is a new, sophisticated form of cellular immunotherapy designed to target cancer cells by leveraging the body’s immune system using specialized cells called T-lymphocytes. T-lymphocytes help destroy tumor cells and control immune responses by preventing the immune system from attacking the body’s healthy cells.
Certain cancer treatments leverage T-lymphocytes from a patient’s own body to infiltrate the tumor and attack the cancerous cells. By amplifying these cells into the billions and delivering them back to the patient, these cells are able to target cancer cells.
Lifileucel (AMTAGVI) is the first cellular therapy for solid tumors using T-lymphocytes that has been approved by the U.S. Food and Drug Administration (FDA). Currently AMTAGVI TIL therapy is approved for use in patients with metastatic melanoma, and it is also being investigated for use in other solid tumors such as lung cancer, head and neck cancers, sarcoma, and cervical cancer.
Weill Cornell Medicine and NewYork-Presbyterian Hospital offer this innovative TIL treatment option to patients. Additionally, researchers at Weill Cornell are leading efforts to help expand TIL therapy and other cellular immunotherapies to improve outcomes for patients with melanomas and different forms of cancers.
“TIL is a practice changing treatment that is a new standard of care for patients who have progressed on prior immunotherapy,” said Dr. Barbara Ma, a medical oncologist caring for patients with skin cancers at Weill Cornell Medicine/NewYork-Presbyterian Hospital. “We are proud to offer this novel treatment to our patients.”
TILs are formed from lymphocyte cells, a form of immune cell that helps the body fight infections and diseases. There are two main types of lymphocytes called B lymphocytes and T lymphocytes. B lymphocytes produce antibodies and T lymphocytes, also known as T cells, are responsible for helping the immune system to fight off germs and protect the body from diseases, such as cancer.
When cancer is detected, the body naturally creates TIL cells to try and fight off the cancer, but as cancer progresses, the body’s natural cells can no longer keep up with the rapidly dividing cancer cells. TIL therapy helps build on this natural process occurring in the body by collecting unique cells from the patient’s tumor and enhancing and expanding them in the laboratory, creating many more multiples of these TIL cells beyond what the body can naturally produce. Because these TIL cells are naturally found in the body, they already have the ability to recognize cancer cells, making this form of treatment effective at attacking the cancer.
TIL therapy is a one-time treatment consisting of several steps. After the tumor is removed from the patient, it takes approximately 1 month to make the specialized cells, followed by a multi-step process to prepare the body for the treatment, receive the TIL infusion, and receive post-infusion treatment and monitoring. The entire process can take several weeks from start to finish.
In order to create the copies of these tumor fighting TIL cells, a small piece of a patient’s tumor is removed by a surgeon. These surgeries can often be done as outpatient procedures.
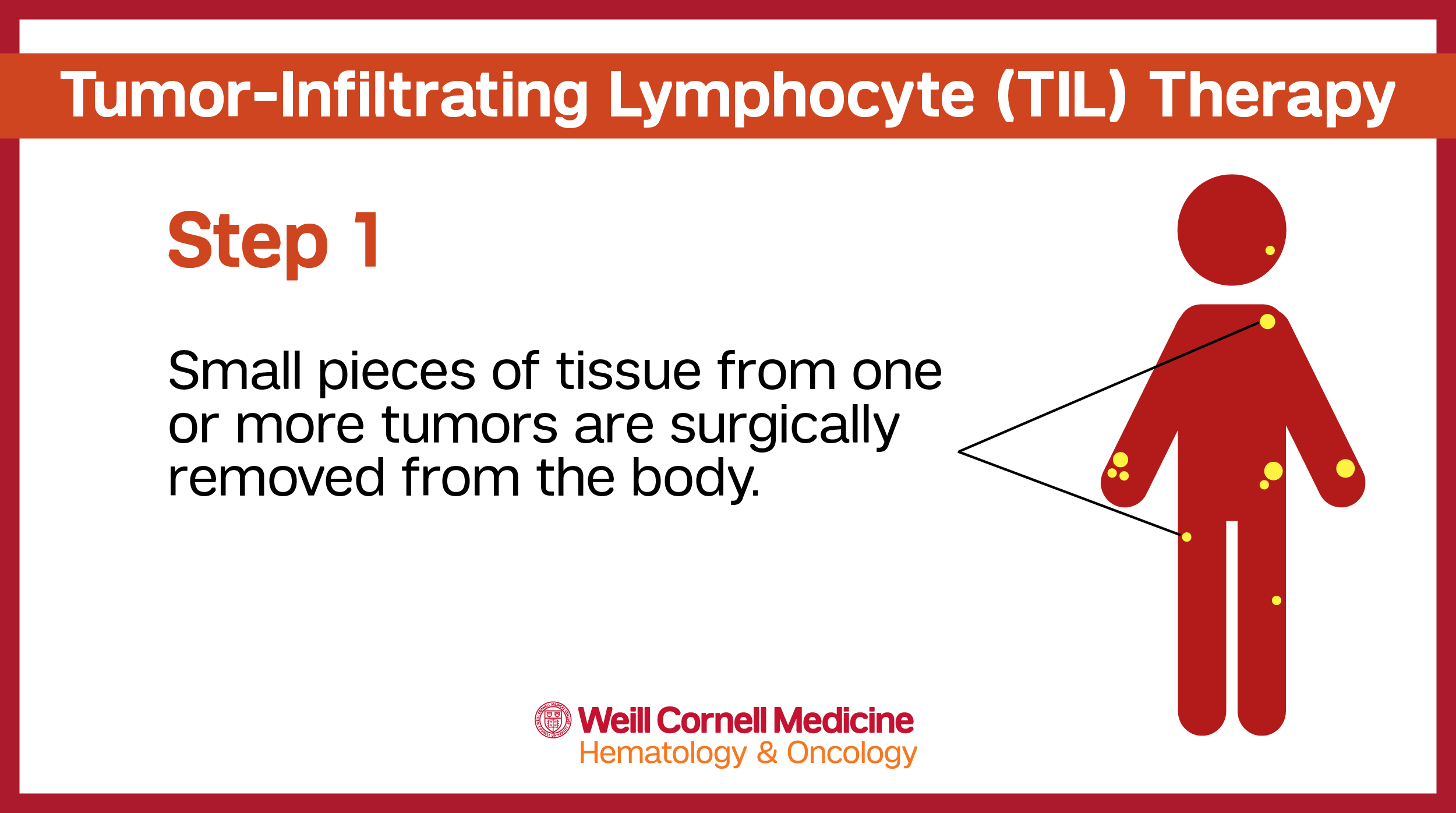
The tissue from the tumor is used to collect the t-lymphocyte cells that will then be used to create individualized TIL therapy – unique to each individual patient and their specific tumor.
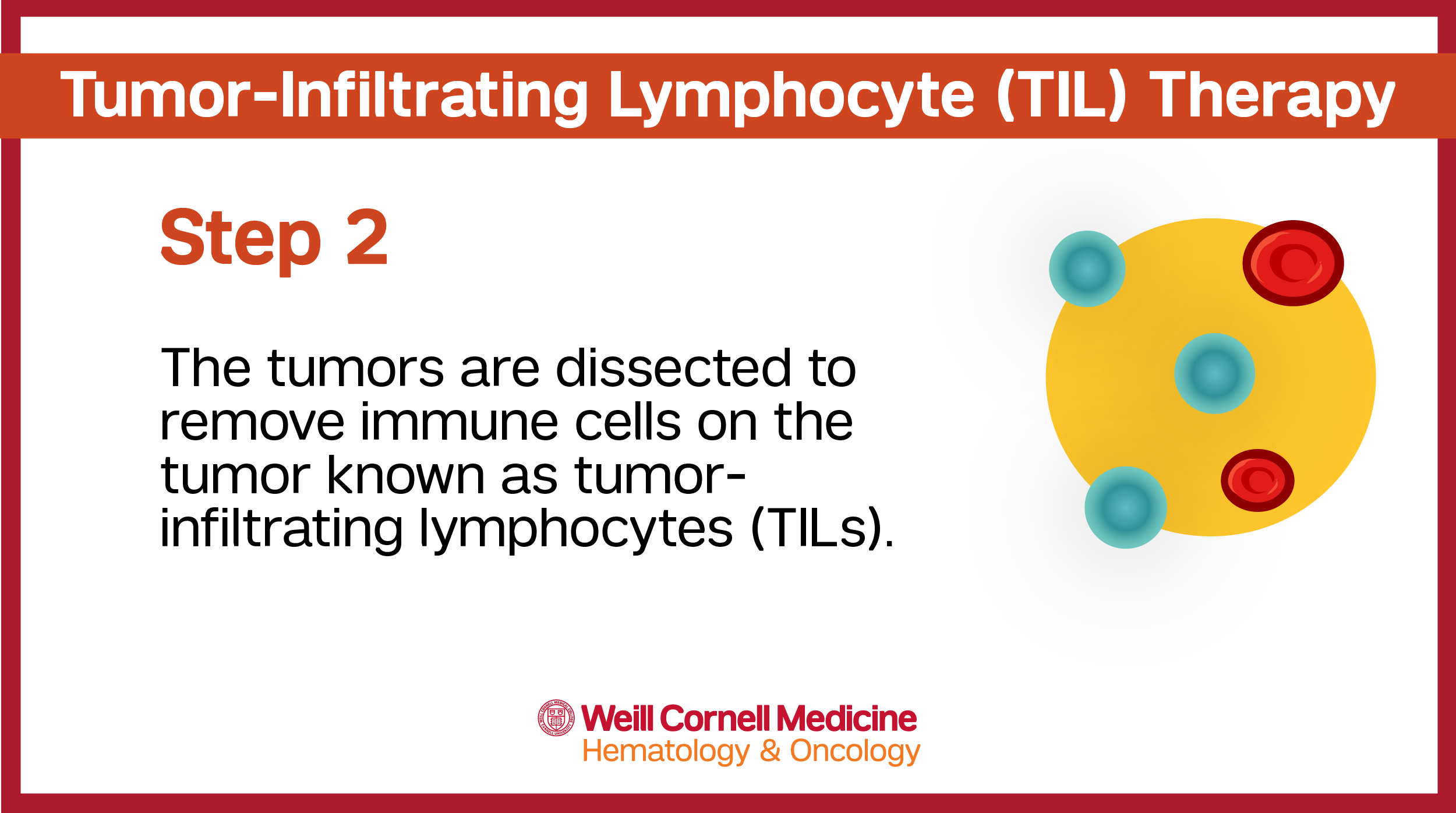
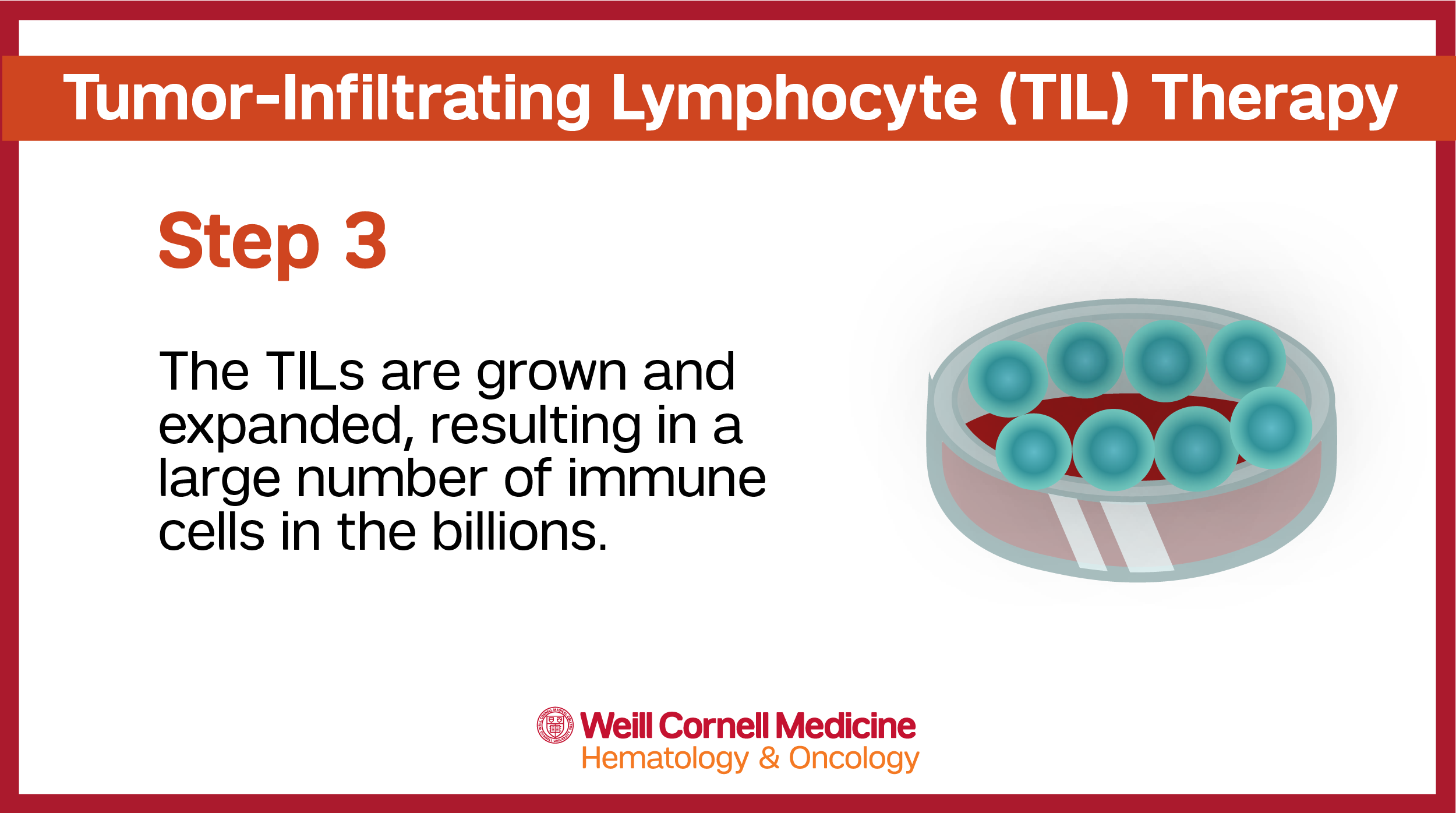
The tissue is then sent to a specialized manufacturing facility where the T cells are separated from other cells. These cells are then rejuvenated, grown and multiplied before being sent back to the care team to be administered to the patient via an intravenous (IV) infusion.
Prior to the TIL therapy infusion, patients will be admitted to the hospital to receive a short course of chemotherapy (starting 7 days beforehand). This helps to create space in the body for the TILs and prepares the body to receive the treatment more effectively. Because chemotherapy can affect the immune system, receiving it prior to the TIL therapy improves the likelihood that the TILs will survive when they are infused into the body.
Patients will stay in the hospital after completing chemotherapy. Twenty-four hours later, patients will receive the TIL therapy via IV infusion, which typically takes less than 90 minutes.
Following the TIL treatment infusion, patients will receive a short course of interleukin-2 (IL-2) therapy to support T cell activity in the body. IL-2 is a medicine that helps expand and activate the number of TILs in the body. Because a large number of TILs enter the body during the TIL therapy infusion, IL-2 is an important step in boosting response. This process can take up to 3 days.
Patients are closely monitored throughout this process for any side effects or reactions to the medication. Common side effects can include chills, fever, fatigue, and shortness of breath. This is due to the nature of the way the treatment activates and “jump starts” the immune system.
A variety of care team members are involved in the TIL therapy process. An oncologist, surgeon, cell therapy coordinator, nurse navigator, and social worker may all be part of the team assisting patients as they undergo this treatment. Ask your care team any questions you may have about the process or the team members who will involved in your care.