Cancer researchers have worked for decades to develop medications that boost the body’s own immune system’s ability to fight cancers. This treatment approach, called immunotherapy, has become an integral part of treating many tumor types and is gaining further traction in breast cancer.
“We know cancer develops from some of our normal cells when changes or alterations occur that lead to abnormal cell growth and spread. A key question has always been: Why doesn’t our immune system recognize that something went wrong in those cells and help us eliminate it?” said Vered Stearns, MD, who leads the Weill Cornell Medicine (WCM) Breast Medical Oncology team and directs the breast cancer translational research program, which aims to develop new treatments in the laboratory and ultimately bring them to patients.
The immune system is the human body’s natural ability to identify and destroy abnormal cells that cause disease, from the common cold to cancer. Yet the very nature of a cancer cell is to rapidly divide and multiply, while learning ways to dodge the immune system in order to do so. Cancer cells begin as normal healthy cells in the body, which makes them more difficult for the immune system to detect since they don’t originate from outside the body. As cancer begins to grow and spread, these cells can adapt to further avoid detection from the immune system, in addition to developing ways to proliferate even more rapidly.
Patients often wonder: why can’t the immune system recognize breast cancer cells as invaders in the same way the body might recover and heal from a virus like influenza?
In the last two decades, there has been a revolution in targeted cancer drugs that support and leverage the body’s own immune system. For certain tumor types like melanoma, bladder and lung cancers, immunotherapy has changed the treatment and patient-care landscape.
Immunotherapy treatments often result in fewer side effects, and particularly those frequently associated with treatments such as chemotherapy and radiation. Immunotherapy is usually better tolerated by patients, with side effects more closely linked to how the drugs impact each person’s immune system.
Breast cancer has been more resistant to immunotherapy drugs, but in 2021 the Food and Drug Administration (FDA) approved the first immunotherapy treatment for a breast cancer subtype called triple-negative breast cancer (TNBC). The drug, pembrolizumab, can be combined with chemotherapy or other standard treatment approaches in some patients with early stage and metastatic disease.
Yet despite improved survival for some, not all patients benefit or even qualify to receive immunotherapy based on the distinct features of their tumors.
Triple-negative breast cancers tend to be aggressive and lack the three receptors more commonly found on breast cancer cells – estrogen, progesterone and HER2 protein – which is how the “triple-negative” name is derived. New therapies are urgently needed to help patients with TNBC live longer.
“My conversations with my patients are critical for me to understand their needs. I work with researchers to try to identify new solutions for patients to live longer and survive their cancer. The goal is to incorporate the best possible standard of care with opportunities for clinical trial participations for our patients,” said Dr. Stearns.
Ongoing research at Weill Cornell Medicine and elsewhere continues to suggest that some women can live with their cancer much longer when treated with immunotherapy.
“There have been situations where we have been able to eliminate cancer completely with medications that target the immune system, so we’re optimistic the future will give us more opportunities to do so,” said Dr. Stearns.
The WCM Breast Center is embarking on an initiative to develop next-generation treatments and personalized approaches to help more TNBC patients benefit from immunotherapy. This work includes identifying new biomarkers to predict how aggressive TNBC tumors will respond to the treatment, as well as testing new combinations of drugs to determine the next generation of standard treatment.
Dr. Stearns, in conjunction with fellow members of the WCM Breast Center team, prioritizes transforming laboratory research findings to develop clinical trials for patients, which can result in new and improved treatment options that ultimately become the standard of care.

Ashley B. Schreier, DO and Roberta Zappasodi, PhD
Recent findings led by medical oncologist Ashley B. Schreier, DO, and research scientist Roberta Zappasodi, PhD, stemming from the WCM Zappasodi Lab, will build off the discovery that tumors such as TNBC metabolize sugar primarily through a process called glycolysis, which can decrease the body’s immune response. Specifically, this tumor metabolic process makes certain immunosuppressive T cells, called regulatory T cells (Tregs), thrive in the tumor bed. Finding ways to counteract this process could improve and optimize the immune system to work harder against TNBC cells.
“Based on the work of Drs. Schreier and Zappasodi and my previous research, we have developed a new clinical trial for patients where we will combine two agents (checkpoint inhibitors) that target the immune system,” said Dr. Stearns “This is an outgrowth of prior immunotherapy research that suggests there are patients with TNBC who are very likely to benefit from this category of medications which help the immune system better recognize and attack cancer cells.”
Working together, these medications target different areas of the immune response – by depleting Tregs and simultaneously blocking two main immune checkpoints (PD-1 and CTLA-4) – to suppress cancer growth and progression. Through this new study, the WCM Breast Center hopes to identify new treatments for both advanced and early-stage TNBC, gaining a clearer understanding of who is most likely to benefit. In the future, this might mean combining immunotherapy agents with chemotherapy or other standard approaches used today, or it could be a combination of more than one agent that targets the immune system.
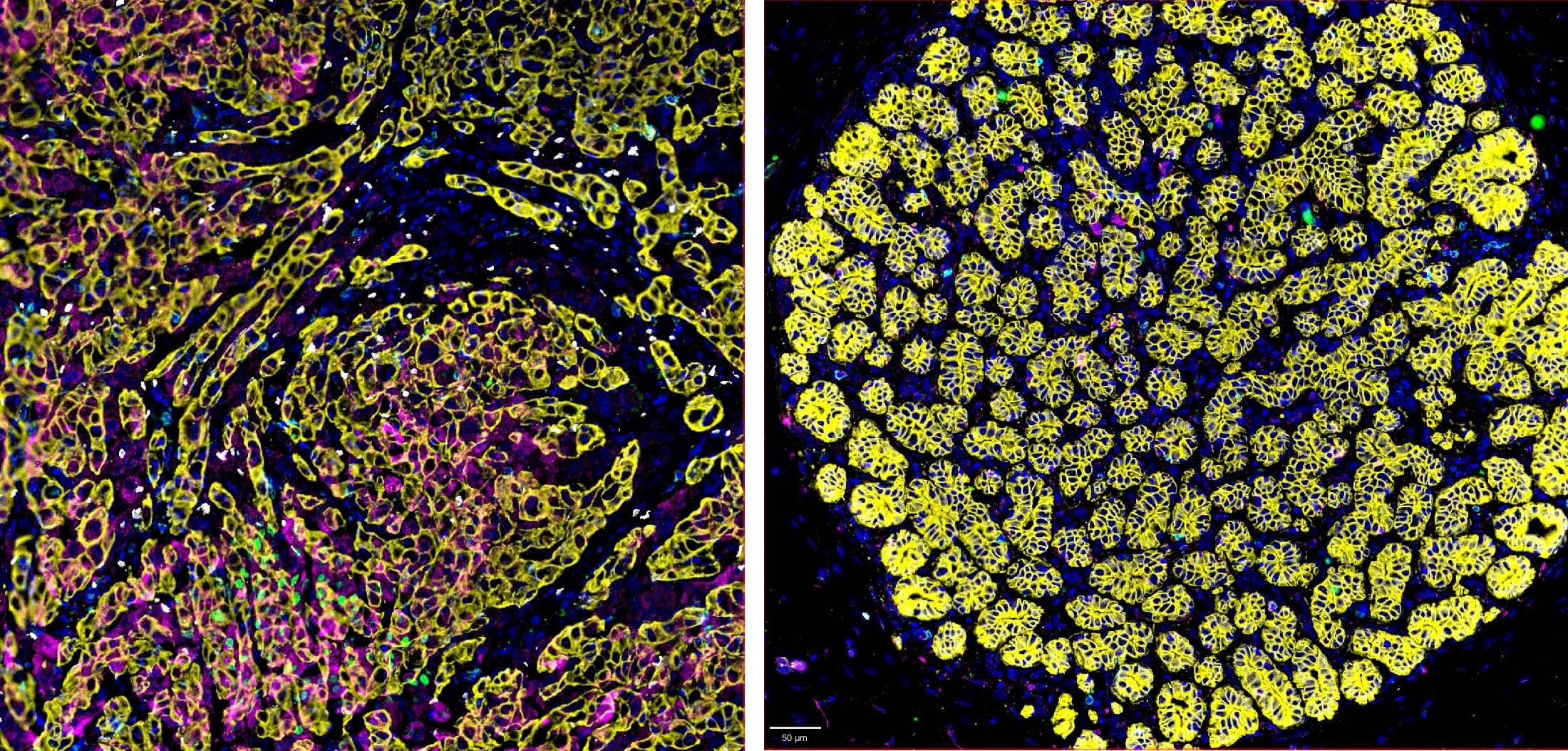
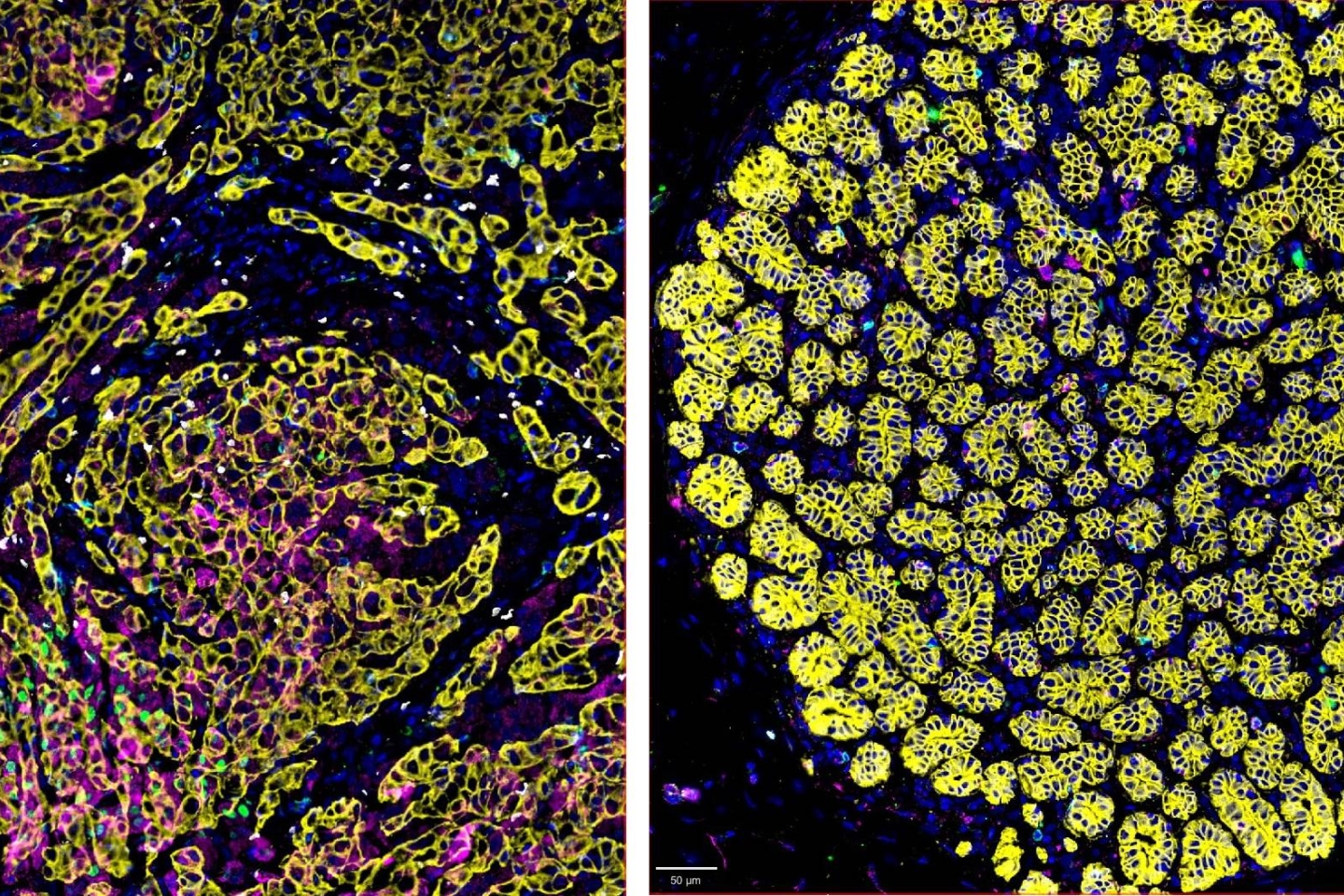
The TNBC cellular microenvironment is complex. Immunosuppressive regulatory T cells (Tregs) abundantly infiltrate TNBC tissue (left) compared with healthy nearby breast tissue (right).
In addition, the team is working to determine metabolic biomarkers that can anticipate the response to immune checkpoint blockade therapy with and without Treg depletion therapy. The hope is that this information will ultimately guide the use of these immunotherapy agents for treating patients with breast cancer that are expected to derive the greatest benefit.
“For the near future, the goal is to help patients live longer with their cancers and live well with their cancers, to provide treatments with fewer side effects, and help our patients enjoy daily activities,” said Stearns, who recalled one patient from a past immunotherapy clinical trial with metastatic TNBC whose cancer disappeared almost immediately and went back to living her normal life.
Success stories like this fuel research and discovery, with each new revelation building upon the potential for immunotherapy treatment to benefit more patients with breast cancer.